Preparative disk gel electrophoresis for antigen preparation
![]() Preparative disk gel electrophoresis for antigen preparation
Preparative disk gel electrophoresis for antigen preparation
| Proteins from mammals and plants are often overexpressed as inclusion bodies
in Escherichia coli. When specific antibodies are prepared, antigens should be purified from
the inclusion bodies. In a simple method for antigen preparation, inclusion
bodies are often washed with detergent-containing buffers such as Triton
X-100 (ref. 1). However, sometimes when recombinant proteins are expressed in E. coli at low levels, the purity of the target protein is not enough. In such
cases, further purification of inclusion bodies by preparative disk gel
electrophoresis is a useful method forantigen preparation (ref. 2). Preparative disk gel electrophoresis has several advantages in antigen preparation from inclusion bodies.
|
|||
| ref. 1) Harlow, E. and Lane, D., Antibodies: a laboratory manual (1988), CSHL press. ref. 2) Okegawa, Y., Koshino, M., Okushima, T. and Motohashi, K., Protein Expr. Purif. vol.118, 77-82 (2016) ---------------------------------------------------------------------- |
|||
| More information is provided in the following references. ref.2 Yuki Okegawa, Masanori Koshino, Teruya Okushima and Ken Motohashi "Application of preparative disk gel electrophoresis for antigen purification from inclusion bodies" Protein Expr. Purif. 118, 77-82 (2016) |
|||
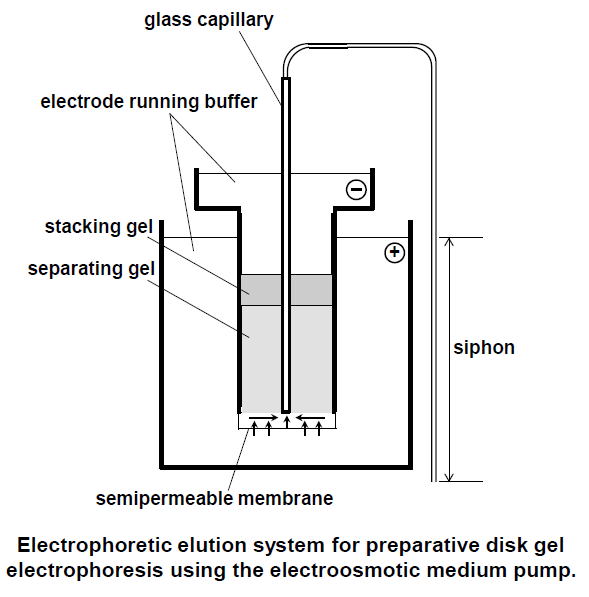 |
|||
| Experimental example 1) ・Acetyl-CoA carboxylase (~50 kDa) ・Acetyl-CoA carboxylase could be purified from inclusion bodies washed with Triton X-100. Contaminat proteins from E. coli were removed by preparative disk gel electrophoresis. ・A specific Acetyl-CoA carboxylase antibody could be produced using antigen purified by this method (ref. 2). |
|||
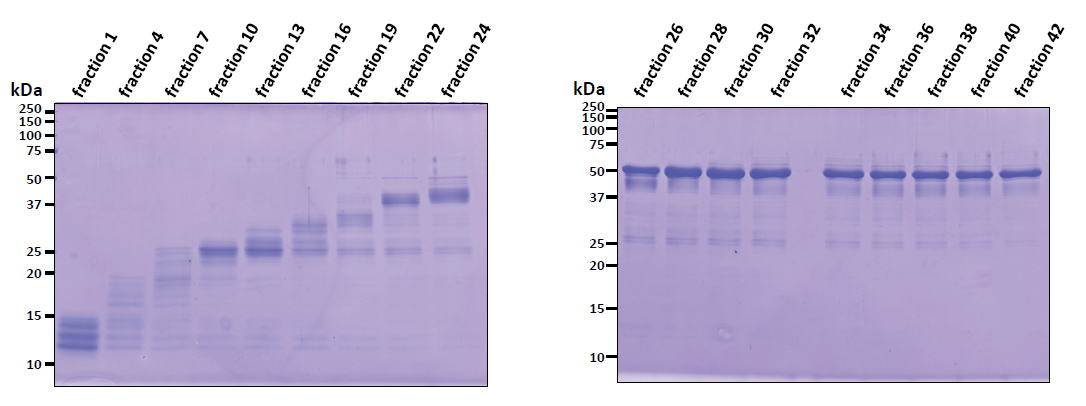 |
|||
| Experimental example 2) ・~15 kDa. ・E. coli contaminant proteins were removed from the 15 kDa target protein by preparative disk gel electrophoresis. |
|||
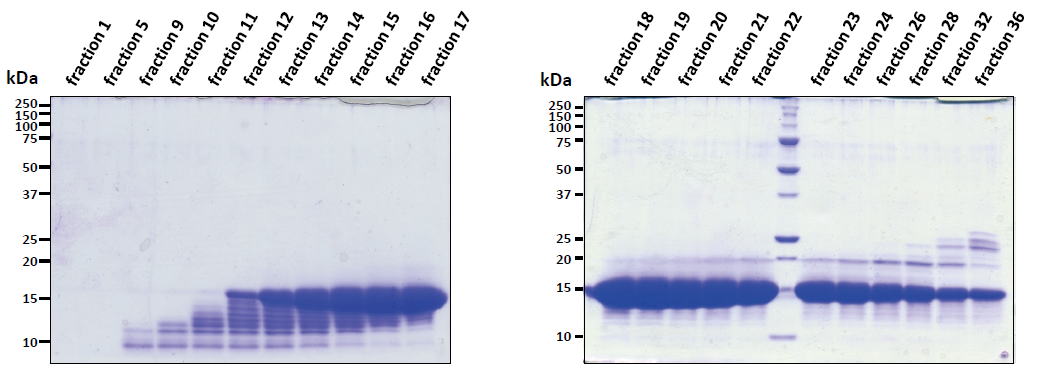 |
|||
  |
 |
||||
| Return to "Technical tips" | ||||
| E-mail: |