Professor Misuzu K. Seo
 ■Area and Subject Taught:Molecular and Cell Biology, Cell Signaling
■Area and Subject Taught:Molecular and Cell Biology, Cell Signaling
■Research Theme(s):Regulation of Cell Proliferation and Differentiation by
Growth Factors and Cell Signaling
■Academic Degrees:Ph. D. (Pharmaceutical Sciences)
■Keywords for Research Field:Cancer Molecular Target Therapy,
Angiogenesis, Neurogenesis, VEGF, FGF, Kallmann syndrome, Anosmin-1
[Research Overview]
Cancer Molecular Target Therapy- Angiogenesis
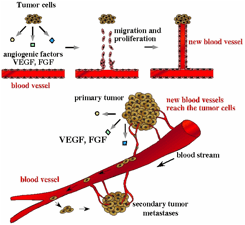
Why do cancers become malignant (likely to cause death) and spread (metastasize) around the body? Because cancer cells secrete cell proliferation factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) that cause vascular endothelial cells to congregate in cancer tissue and induce new blood vessels (angiogenesis). As a result of being supplied with fresh nutrients and oxygen via these blood vessels, the cancer cells undergo dramatic growth and infiltrate the blood vessels, spreading to different organs. We are investigating the molecular mechanisms whereby cancer cells secrete VEGF and FGF and stimulate endothelial cells to form new blood vessels into the cancer tissue. Specifically, our research focuses on the question of whether or how VEGF and FGF help endothelial cells and cancer cells to survive and proliferate, and how signals (information) are transmitted to the nucleus after angiogenesis factors are accepted by receptors on the cell surface. In addition, VEGF and FGF induce the survival and differentiation of neurons. Our contributions in these research fields are expected to lead to the development of regenerative therapies, which are currently the center of attention, as well as novel cancer treatments.
Molecular Neuroscience- Kallmann syndrome
 Recently, the Seo lab has been studying the molecular mechanisms of a genetic disease called Kallmann’s syndrome (KS), which affects 1 in 10,000 males and 1 in 50,000 females. The phenotypical features of KS are infertility and inability to smell, which are due to the impairment of the migration of gonadotropin-releasing hormone (GnRH) neurons and of olfactory axon development. The two responsible genes for the disease are KAL-1 (anosmin-1) and KAL-2 (fibroblast growth factor receptor 1: FGFR1). We therefore analyzed the functions of FGFR1 and anosmin-1 using PC12 cells as a neuron model in culture. We showed that PC12 cells expressing normal FGFR1 upon stimulation of FGF2 developed neurite outgrowth (red) and that anosmin-1 greatly enhanced the formation of growth cones (green). We are under investigation how the extracellular signals from FGF2 and anosmin-1 are conveyed to the intracellular proteins to form connections between neurons and target cells. Our work will provide insight into the molecular mechanisms controlling the development of the central nervous system.
Recently, the Seo lab has been studying the molecular mechanisms of a genetic disease called Kallmann’s syndrome (KS), which affects 1 in 10,000 males and 1 in 50,000 females. The phenotypical features of KS are infertility and inability to smell, which are due to the impairment of the migration of gonadotropin-releasing hormone (GnRH) neurons and of olfactory axon development. The two responsible genes for the disease are KAL-1 (anosmin-1) and KAL-2 (fibroblast growth factor receptor 1: FGFR1). We therefore analyzed the functions of FGFR1 and anosmin-1 using PC12 cells as a neuron model in culture. We showed that PC12 cells expressing normal FGFR1 upon stimulation of FGF2 developed neurite outgrowth (red) and that anosmin-1 greatly enhanced the formation of growth cones (green). We are under investigation how the extracellular signals from FGF2 and anosmin-1 are conveyed to the intracellular proteins to form connections between neurons and target cells. Our work will provide insight into the molecular mechanisms controlling the development of the central nervous system.